Do you have a great idea for a medical device and need a partner to realize it and apply a CE mark at a notified body? Read through some challenges you will encounter developing and approving medical devices. Learn what to look out for in terms of R&D, quality and regulatory capacity in the CE certification medical devices.
BAAT is an experienced partner and expert in medical device development. We have the experience and know-how to transform your dream, idea, technology or problem into a product with manufacturing route, CE mark and supply chain management.
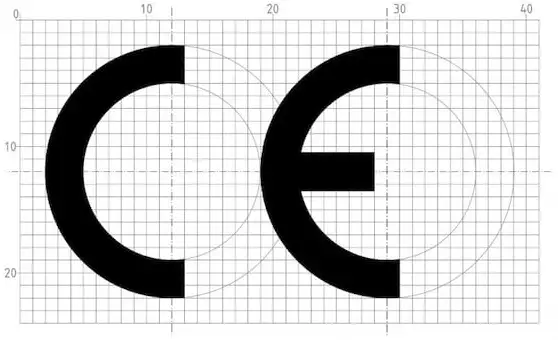
You find the CE mark on toys, electronics, protective clothing, and many other products sold in Europe. CE marking was introduced to support the EU’s goal of removing trade barriers within the EU. Originally, products with possible safety, health or environmental issues had to be approved by each country. Now, national legislation is harmonized into Europe-wide directives or regulations; if manufacturers guarantee both the product and testing is conform these directives, they have access to all EU countries at once. Manufacturers must put the CE mark on their products to signal they conform to the applicable European directives.

Did you know that as a manufacturer, you must officially declare that your product conforms to the CE mark medical device requirements? This declaration requires technical documentation, to support your ‘case’ in that your development process, and resulting medical products, are manufactured and tested conform all regulations.
Medical devices are divided into different risk classes. For the lowest risk class manufacturers may self-certify. Meaning they themselves can draw the conclusion that the technical documentation proves conformity with the regulations and put a CE mark on it.


For medium or high-risk medical devices, conformity with the regulations cannot be done by the manufacturer; a Notified Body must assess conformity before it can be placed on the market. What is a notified body in CE marking? These are organisations designated by member states of the EU who can assess conformity with specific regulations.
Medical products can only be assessed by organisations with a notified body certification specifically for assessing medical devices. The number of the Notified Body involved in the assessment must be shown; an implant assessed by the Notified Body MedCert will show ‘CE 0482’ and shown here is an example of Dekra ‘CE 0344’ as Notified Body.
When you transform your idea into an approved medical device, you will encounter hurdles. BAAT is successfully guiding customers to overcome these hurdles since 1999. Contact us today to use this experience to navigate the intricacies of working with a notified body and CE mark medical devices.
As your full-service medical device company, we’re partners for the complete journey and are only satisfied when your medical device is CE marked and successfully treating patients.
Get your medical device CE marked and successfully treat patients.
Asking the right questions is the first step to a successful project. Having impact on the end user means impact in the market. Some examples:
One of the steps many customers do is answering above questions using an Innovation Scan. We call it ‘Innoscan’. Get in contact with Baat to see what can lead to a commercially viable product development and real impact in the medical device business.
Our ISO 13485 quality system (certificates) concerns topics directly related to product development (e.g. verification and validation, risk management, usability), but also addresses many high-level topics such as competence tracking and training, management of standards, control of documents.
When partnering with BAAT, you will not only benefit from the knowledge and experience of our 25+ people with various fields of expertise, our expertise with medical device engineering for both the EU and US markets with 30+ products developed (projects), but also of our underlying quality system.

Business development
"*" indicates required fields